Our New Generation 3 Genesis Bi-Wing AAP® vs Our 2nd Generation Bi-wing AAP®
In the upcoming first quarter of 2024, we will commence the finalization of the transition from our current Generation 2 Genesis Bi-Wing AAP® to our next generation. Kindly review this article to grasp the differences and enhancements we have incorporated into our next-generation platform. The key alterations primarily pertain to the FDA’s evolving human factors requirements .
The Old Genesis Vs the New Generation Genesis Bi-Wing AAP®
Our current Genesis Bi-Wing AAP® is undergoing a transition to the next Generation Genesis Bi-Wing AAP®. Rest assured, there are no quality or efficacy issues associated with this change. In fact, this system represents a significant advancement from our previous generations. We kindly request the opportunity to discuss the differences.
Previous Second Generation Genesis:Â Â Â Â Â Â Â Â Â Â Â
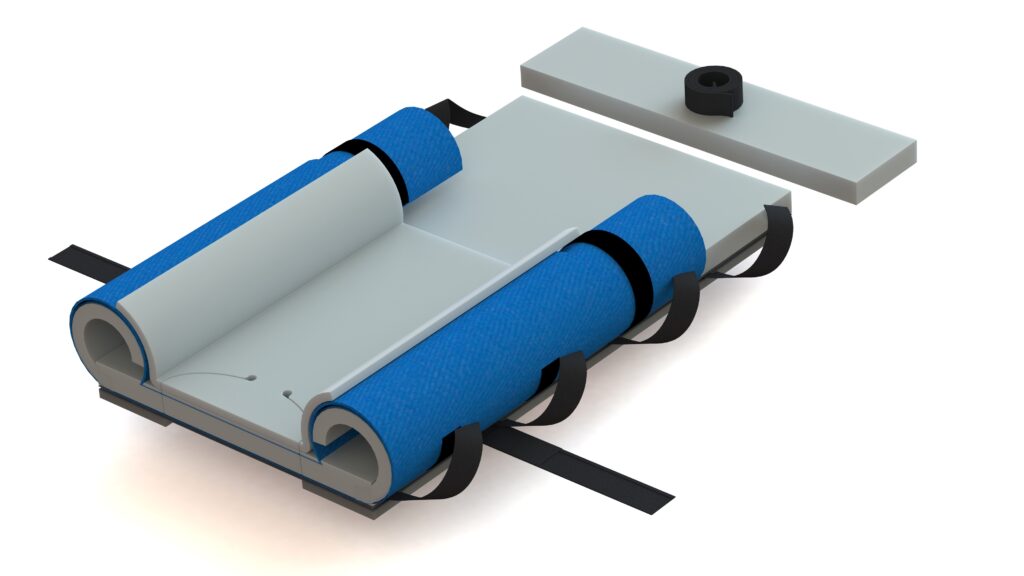
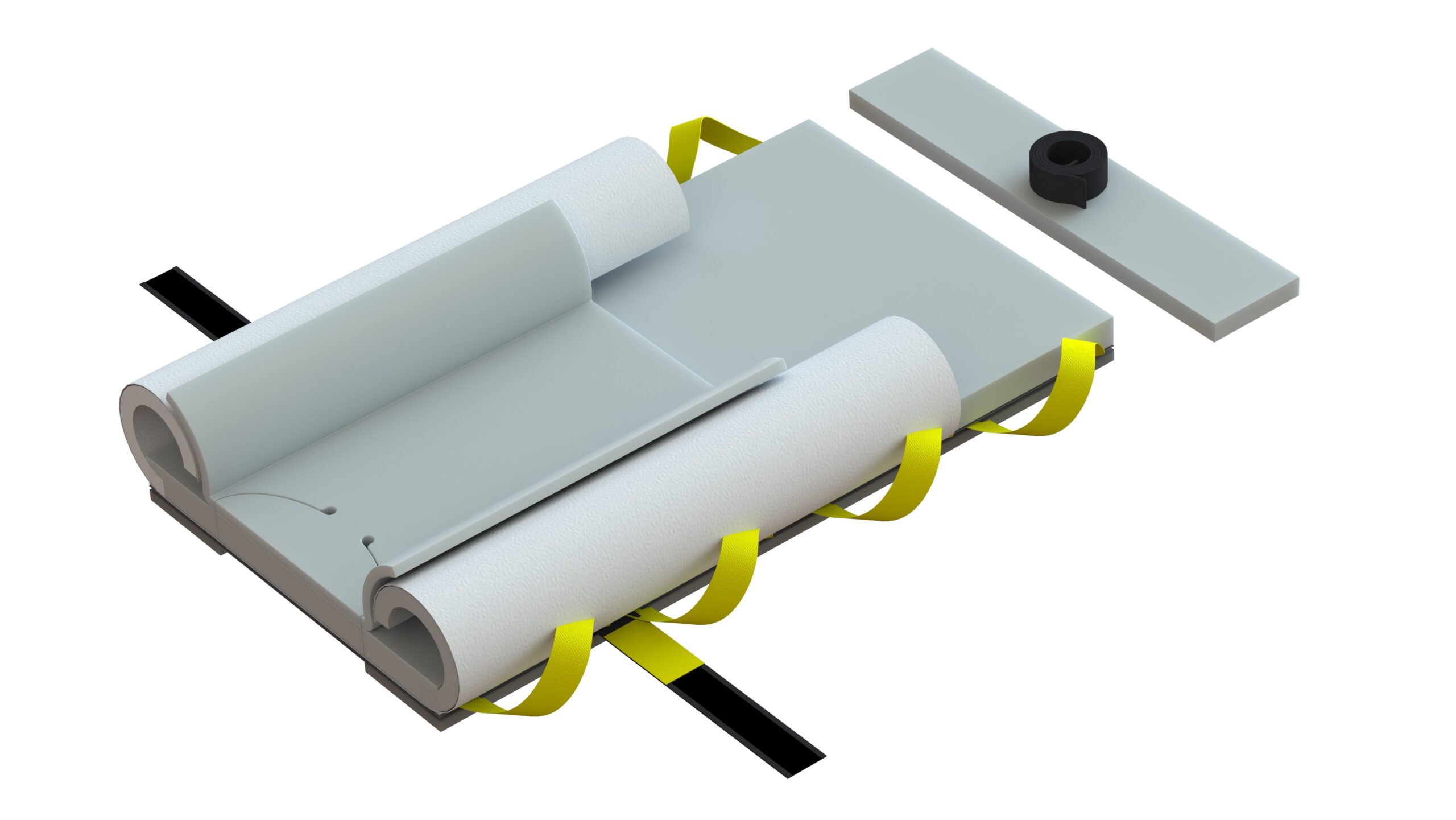
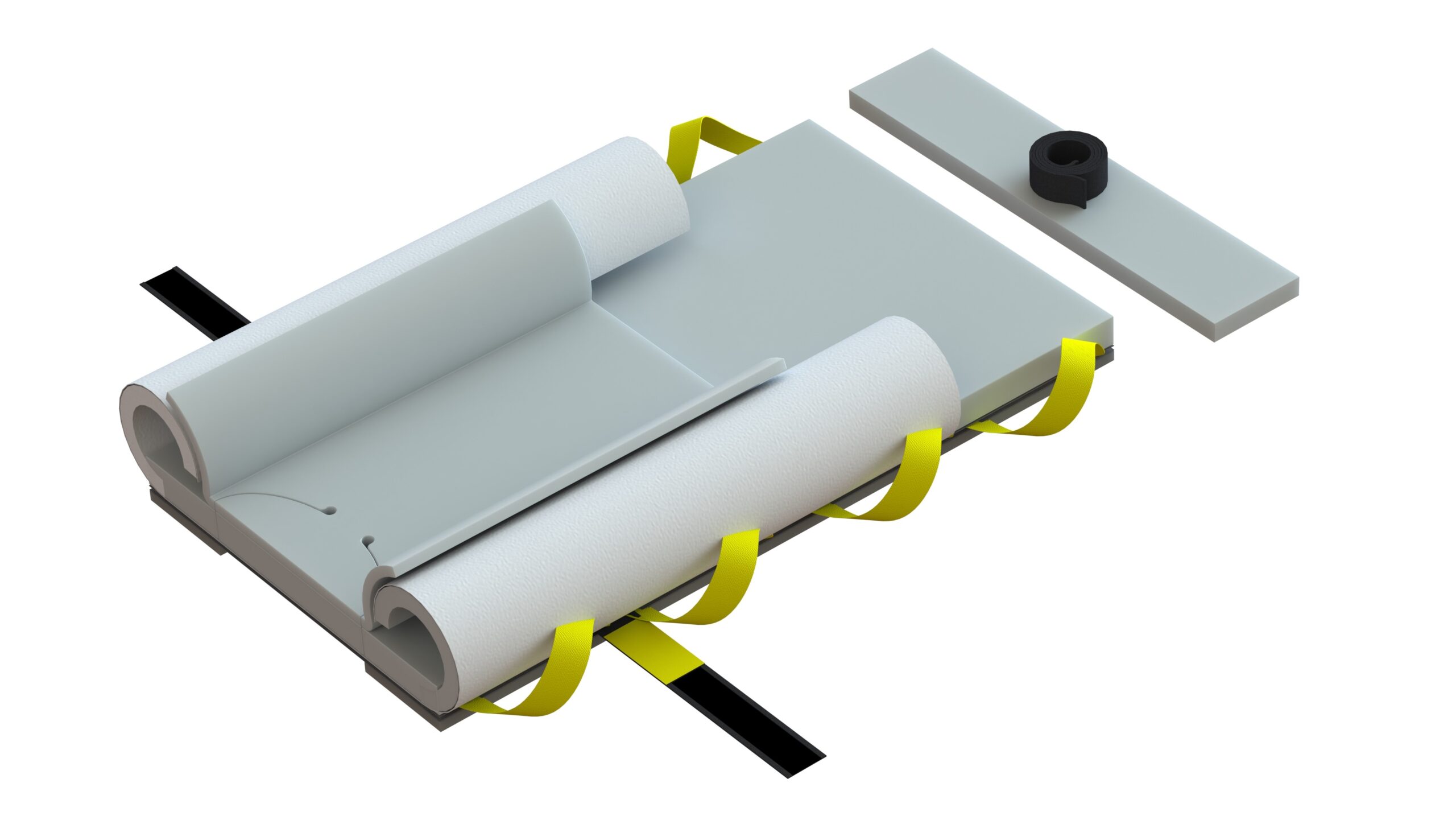
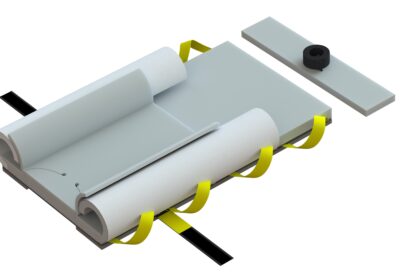
Why does the new Genesis look different? The new Genesis has undergone visual enhancements to ensure safety and compliance with FDA requirements for human factors in product use. These changes include the adoption of safety colors and the incorporation of visual contact point color cues necessary for securing the system to the surgical table’s rail system and the chest strap. Notably, the handles have been redesigned in yellow, providing a clear visual cue for staff to use as many as needed for safely moving the patient. Additionally, the upper wings have transitioned from blue to white, mitigating confusion with similar-colored sterile drapes and reducing the risk of contamination. These improvements align with our commitment to reducing variance and optimizing the user experience, demonstrating our dedication to maintaining the highest standards of safety and efficiency.
Generation 3 Genesis Bi-Wing AAP
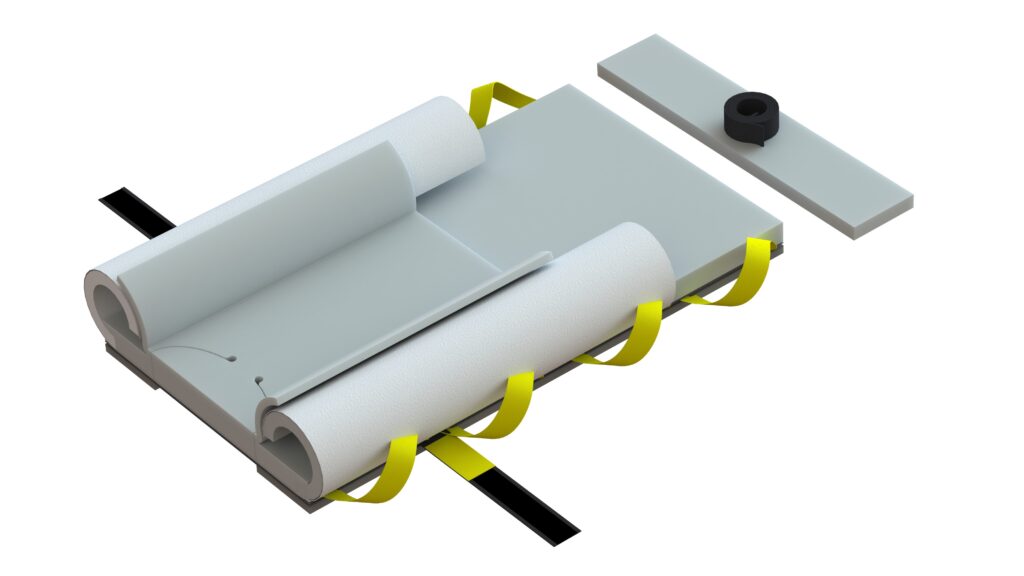
Is there any difference in the foam? No, the foam maintains the same high-quality and thickness as the previous Genesis series. Our advanced Pneumafom® sets us apart from typical memory foams used by our competitors. Its ILD (Internal Load Deflection) quality ensures superior pressure management and reduces the occurrence of “bottoming out” often experienced with other 1-inch memory foam pads. Rest assured, our Genesis base pad and chest pad have always been 1.5 inches in height.
Why the material changes? The decision to transition from SMS non-woven materials to nylon based Taffeta material was driven by the evolving requirements for environmental sustainability from our customers. This shift allows us to reduce the presence of microplastics during breakdown, which is a common concern with SMS non-woven materials, including surgical drapes and gowns. Importantly, this change does not compromise the quality of our products. In fact, the new material is five times stronger than our previous layered SMS non-wovens, resulting in a lighter system with only two sheets instead of four. This enhanced material change does not impact efficacy or quality. Our systems are rigorously tested and can withstand a free hang capability of 1500 lbs., making them compatible with mechanical lifts via our lift bar systems.
Why the foam strip changes on the bottom? We have improved the friction coefficient of the new foam strips on the bottom, and slightly widened their placement to facilitate patient movement during lithotomy required positions, thereby reducing the required lift clearances for staff. These enhancements do not compromise the product’s integrity. In fact, they are specifically designed to offer lateral stability during bed rotations to the right or left, as our current straps and reinforced pads can withstand significant force loads in directions like Trendelenburg (head down).
Regarding the number of straps used to secure the pad to the table, many customers inquire about the absence of four straps when compared to our competitors. The reason for this is the unique construction of our fused foam pads to the lift carrier. Unlike our competitors, who only attach straps to unreinforced foam through gluing or stitching, their systems often stretch and tear during use, leading to pad and patient migrations during procedures. In contrast, our fused pads do not stretch or tear during use due to their construction, ensuring superior performance. The two anchor straps provide the necessary strength for the entire system during head-down rotations, while the chest strap secures the upper part of the pad and provides the required lateral stability for both head-down and lateral bed rotation changes necessary for colorectal procedures.
It is noteworthy that human factor behaviors have consistently been incorporated into our designs. Through our 20 years of clinical practice, we have observed that the inclusion of four foam straps in unreinforced foam has led some individuals to believe that a chest strap is unnecessary. However, it is imperative to emphasize that chest straps are absolutely essential for any product, particularly when there is lateral rotation involved. In order to ensure proper placement and use of the chest pad and minimize the potential for off-label use or safety risks to patients, we have intentionally omitted the two upper anchor straps.
For additional information or educational resources on this topic, we invite you to watch our video: https://vimeo.com/892396017?share=copy
As a company rooted in clinical expertise, we are committed to continuously improving our products based on customer feedback and the evolving demands of surgical care and safety. We are grateful for your continued support and for the opportunity to contribute to your exceptional surgical care. Always here to answer any comments or concerns!
Sincerely,
David Gomez CRNA
CEO
Infinitus Medical Technologies